From safety and security perspectives, the importance of tracing distribution process of objects (traceability) has significantly increased.
YRP UNL has conducted the fundamental research for identifying individual objects by means of ucode so that attributes of used standards, lot numbers, shipment origins/destinations and installation locations can be registered and managed. Some applications have been put to practical use.
Case StudyⅠ: The Management of Historical Information of Houses
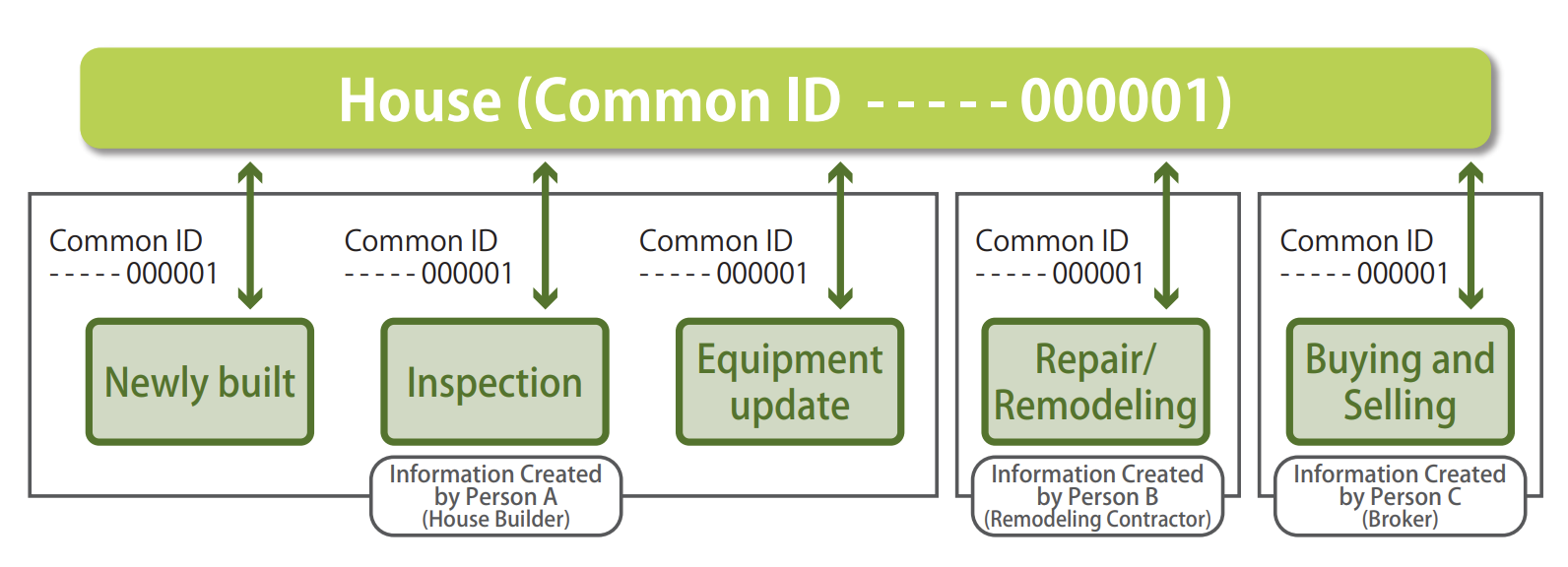
In order to keep using a house for many generations, effective use of past records of the house (historical information of a house) over a prolonged period of time is important for the equipment update, repair/remodeling, and buying and selling.
As an effort to realize this objective, the Historical Information of House Accumulation/Utilization Promotion Council was established. In the Council, ucodes are used as “Common IDs” for identifying individual houses uniquely. The information of past records of the houses are linked with this ID and are managed (Figure 2).

Case Study Ⅱ: Traceability Management System of Housing Components (Better Living)
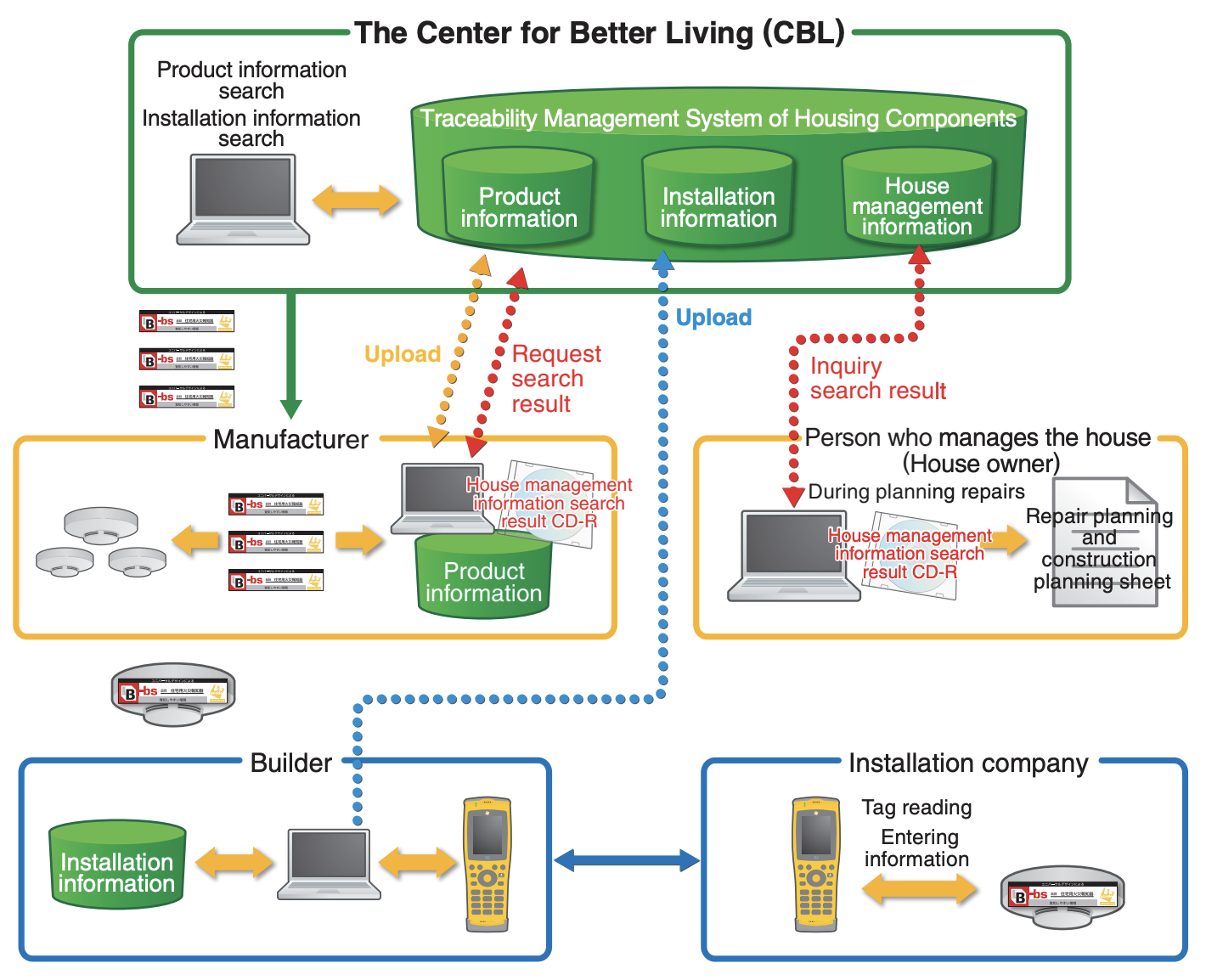
“Who built this house? What kind of housing components are used? Who installed these components? And how were they used and examined?” The Center for Better Living is operating “Traceability Management System of Housing Components” that can resolve those concerns of dwellers (Figure 3). Individual residential fire alarms can be identified by ucode linked with the records of manufacturing, installation and inspection.

Case Study Ⅲ: Ubiquitous Food Traceability System
Food traceability system is a mechanism that records information at each stage of production, processing, and distribution of food, and in case of a problem, immediately identifying the origin of the problem, and extent of the impact from the recorded information.
Such a system enables us to continuously track items with ucode to identify food items/products or lots during production, processing and distribution. This tracking continues even if the mode of packaging changes during the distribution process.

Case Study Ⅳ: Medical Traceability System
Pharmaceutical Affairs Law has been revised, and is now called “Act on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices”. The new law mandates the management of sales records and use history including the names of pharmaceutical formulation, used standards, and lot numbers, at pharmaceutical manufacturers, wholesalers and medical practice sites. It also specifies the minimum archive duration. This revision aims at the post marketing safety measure enforcement of biological products.
Individual medical products can be identified by ucodes. Recording traceability information using RFID Tags can lead to increasing the safe and secure supply of medical products, and is expected to contribute to the streamlined effective distribution of medicines and prevention of medical errors.